Abstract
Introduction: According to international guidelines the vaccination schedule for hematological patients is mainly recommended prior to treatment start, three to six months after chemotherapy and following both allogeneic and autologous transplantation due to weakened immune response with variable response efficacy. As for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus several studies have demonstrated high risk of severe infections in patients with hematological malignancies and prolonged virus persistence.
Methods: Our prospective study purpose was to evaluate the level of COVID-19 immunity prior to antineoplastic treatment and monitor it until 12 months after transplantation (ASCT group) or 6 months following standard chemotherapy treatment (Chemo group) in previously vaccinated patients with confirmed immune response.
All lymphoma and multiple myeloma (MM) patients aged 18 or above that underwent mRNA vaccination against COVID-19, with a confirmed humoral anti-COVID-19 antibody response (targeted antibody titer greater than 17.8 BAU/ml), and were treated with either standard or high-dose chemotherapy and ASCT were eligible. Patients were allowed to receive the second or third vaccination dose in course of study, if they did not receive it previously, and eventual SARS-CoV-2 infections were registered along with the outcome. The study was approved by the independent ethics committee and patients provided written informed consent.
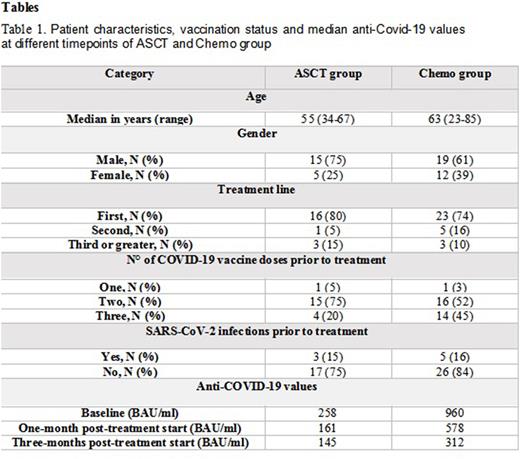
Results: Twenty patients in the ASCT group were monitored with a median follow-up of 6 months (range 1-12) and most of them (80%) were in first line of treatment. Eleven patients had myeloma, with four of them undergoing tandem ASCT, and were, therefore, monitored twice, while four patients were affected by lymphoma. Patient characteristics, vaccination status and median anti-Covid-19 values prior to ASCT, one- and three-months following transplantation are described in table 1. Three patients had COVID-19 infection following ASCT and all of them were paucisymptomatic, with anti-COVID-19 antibody value of 150 BAU/ml, 102 BAU/ml and 50 BAU/ml each. Three patients received the third dose following ASCT, and only in one of them slight increase of anti-COVID-19 antibodies was evidenced. Two patients passed away, one due to disease progression and the second patient had pneumonia-related death not associated to SARS-CoV-2.
Thirty-one patients in the Chemo group had a median follow-up of 4 months (range 0-12), twenty-five suffering from lymphoma (80%), and nineteen of them received rituximab as part of their treatment, while the rest had MM. Table 1 describes in detail the second group, like the ASCT group. Two patients had COVID-19 positivity alone in course of treatment, while one patient had paucisymptomatic infection and had out-hospital treatment, with anti-COVID-19 antibody value of 292 BAU/ml, 60 BAU/ml and 189 BAU/ml each. Five patients received further vaccination dose in course of treatment, without specific humoral response. Three patients passed away, two of them due to disease progression, while one suffered from sepsis-related death in neutropenic patient negative for SARS-CoV-2.
There was no statistical difference according to age, baseline disease, treatment line, number of vaccination doses and rituximab treatment prior to treatment start in the study cohort (p>0.05), using Wilcoxon-Mann-Whitney test. Difference between Chemo and ASCT group anti-COVID-19 values, on the other hand, was statistically different one month following treatment start (p=0.05), unlike baseline and after three months (p>0.05). Lymphoma patients from Chemo group were also more likely to have higher values of anti-COVID-19 antibodies at baseline (p=0.03) and one month (p=0.02) following treatment start compared to MM patients, unlike the ASCT group (p>0.05).
Conclusions: The impact of both standard- and high-dose chemotherapy followed by ASCT on anti-COVID-19 humoral response is described in our study and confirms the immunity loss in treated patients. Furthermore, vaccination in course of therapy or following ASCT showed limited benefit in regaining anti-virus immunity. Therefore, the importance of re-vaccination in patients undergoing induction or consolidation treatment remains doubtful. Further studies with larger patient cohorts and longer follow-up are needed in order to analyze and confirm the abovementioned data.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal